Prices plus VAT plus shipping costs
Ready to ship today,
Delivery time appr. 5-8 days
- Order number: 7TM0319B-PA
- Content: 96-Well Assay Kit (Inquire for bulk orders)
The pT370-MOP Phosphorylation Assay Kit allows determination of MOP Phosphorylation in 96-well plates without the need for Western blot analysis.
- Seed stably or transiently transfected cells on poly-D-lysine (PDL)-coated F-bottom cell culture plates (80.000 - 100.000 cells/well for 96-well plates) and grow overnight to 95% confluence.
- Treat cells for the desired time with agonist, antagonist or inhibitor of interest.
- Prepare cell lysis buffer by dissolving two protease and phosphatase inhibitor tablets into 20 mL Detergent Buffer.
- Aspirate media. Wash wells with ice-cold PBS. Aspirate PBS.
- Add 150 µL ice-cold cell lysis buffer into each well of 96-well plate and incubate on an orbital microplate shaker at 500 – 700 rpm for 30 min at 4 oC.
- Centrifuge F-bottom plates for 20 min at 3,700 x g at 4 oC.
- For parallel detection of phosphorylated and total receptors, lysate is divided during transfer: For detection of phosphorylated receptors and total receptors transfer 60 µL cleared lysate each into corresponding well of 96-well U-bottom assay plate. Thus, splitting of each samples results in two assay plate per cell cultures plate!
- Resuspend Magnetic Bead Solution by vortexing briefly. Then add 40 µL bead solution into each well and incubate for 2 h on a microplate shaker at 500 – 700 rpm at 4 oC.
- Wash microplate three times with PBS with 0.1% Tween 20 (PBST) under magnetic force using a handheld magnetic separation block or automated microplate washer.
- For detection of phosphorylated receptors, dilute Premium Phosphosite-Specific 7TM Antibody solution 1:10 with PBST (0.6 mL antibody solution plus 5.4 mL PBST) and add 60 µL into each well of the 96-well assay plate.
- For detection of total receptors, dilute Premium Non-Phospho 7TM Antibody 1:10 with PBST (0.6 mL antibody solution + 5.4 mL PBST) and add 60 µL into the corresponding wells of the 96-well assay plate.
- Incubate assay plates for 2 h at room temperature or overnight at 4 oC on a microplate shaker at 500 – 700 rpm.
- Wash microplates three times with PBST under magnetic force.
- Dilute Secondary Detection Antibody 1:10 with PBST (1.2 mL antibody solution + 10.8 mL PBST) and add 60 µL into each well of the 96-well assay plates and incubate for 2 h at room temperature on a microplate shaker at 500 – 700 rpm.
- Wash microplates three times with PBST under magnetic force.
- Add 100 µL Detection Solution into each well of the 96-well assay plates and incubate until desired optical density at 405 nm (OD405) is obtained. Ideal dynamic assay range is between OD405 1.2 for positive controls (cells treated with endogenous agonist) and 0.2 for negative controls (untreated cells).
- As soon as desired OD is achieved, which is typically beetween 2 and 6 min, add 50 µL Stop Solution, place microplates onto magnetic separation block and transfer 100 µL of the solution into the detection plate and determine OD405 using a microplate reader.
- 20 mL Detergent Buffer (Cell Lysis Buffer) for 96-well plates
- 2 x Protease and Phosphatase Inhibitor Tablets (dissolve 1 tablet into 10 mL Detergent Buffer before use)
- 8.0 mL Anti-HA Magnetic Bead Solution
- 0.6 mL Premium Phosphosite-Specific 7TM Antibody for detection of phosphorylated receptors
- 0.6 mL Premium Non-Phospho 7TM Antibody for detection of total receptors
- 1.2 mL HRP-Labelled Secondary Antibody for detection
- 20 mL Detection Solution
- 20 mL Stop Solution
- Handheld magnetic separation block (e.g. cat. #VP 771HH-H, V&P Scientific) or automated microplate washer equipped with a magnet (e.g. Biotek 405 LS)
- Microplate centrifuge (e.g. Thermo Fisher Multifuge X Pro, 4,000 x g, 4 oC)
- Multichannel pipette
- Orbital microplate shaker (e.g. Corning LSE digital microplate shaker)
- Microplate reader for measuring OD at 405 nm
- Cell culture plates: poly-D-lysine (PDL)-coated 96-well plates, F-bottom (e.g. cat. #655940, Greiner Bio-One)
- Assay plates: 96-well plates, U-bottom, transparent or white (e.g. cat. #650101, Greiner Bio-One)
- Detection plates, 96-well plates, F-bottom, transparent, (e.g. cat. #655101, Greiner Bio-One)
- Phosphate-buffered saline containing Ca2+/Mg2+ (PBS) and PBS with 0.1% Tween 20 (PBST)
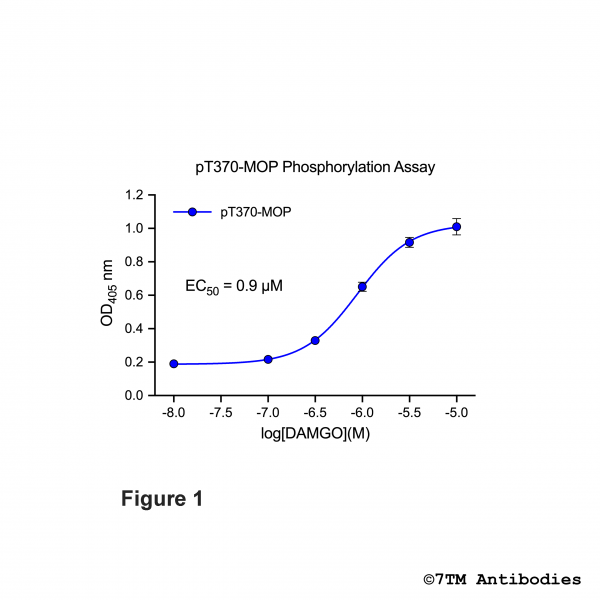
Figure 1. OD signals in pT370-MOP Phosphorylation Assay. HEK293 cells stably expressing the µ-opioid receptor (MOP) were treated with increasing DAMGO concentrations for 30 min at 37°C. Phosphorylation was determined using pT370-MOP Phosphorylation Assay Kit according to the standard assay protocol.
Figure 2. DAMGO-induced MOP phosphorylation determined using the 7TM Phosphorylation Assay. Concentration–response curves were generated after treatment of HEK293 cells stably expressing the µ-opioid receptor (MOP) with increasing DAMGO concentrations for 30 min at 37°C. The cells were then lysed and analyzed for pT370-, pS375-, pT376-, and pT379-MOP phosphorylation according to the standard assay protocol. All data points were normalized to 10 µM DAMGO stimulation.
Figure 3. Morphine-induced MOP phosphorylation determined using the 7TM Phosphorylation Assay. Concentration–response curves were generated after treatment of HEK293 cells stably expressing the µ-opioid receptor (MOP) with increasing morphine concentrations for 30 min at 37°C. The cells were then lysed and analyzed for pT370-, pS375-, pT376-, and pT379-MOP phosphorylation according to the standard assay protocol. All data points were normalized to 10 µM DAMGO stimulation.
Figure 4. Inhibition of DAMGO-induced MOP phosphorylation by naloxone. Concentration–response curves were generated after treatment of HEK293 cells stably expressing the µ-opioid receptor (MOP) with increasing naloxone concentrations for 30 min at 37°C. The antagonist naloxone was added 30 min prior to challenge with 10 µM DAMGO. The cells were then lysed and analyzed for pT370-, pS375-, pT376-, and pT379-MOP phosphorylation according to the standard assay protocol. All data points were normalized to 10 µM DAMGO stimulation.
Figure 5. PMA-induced MOP phosphorylation determined using the 7TM Phosphorylation Assay. Concentration–response curves were generated after treatment of HEK293 cells stably expressing the µ-opioid receptor (MOP) with increasing concentrations of the PKC activator phorbol 12-myristate 13-acetate (PMA) for 30 min at 37°C. The cells were then lysed and analyzed for pT370-, pS375-, pT376-, and pT379-MOP phosphorylation according to the standard assay protocol. All data points were normalized to 10 µM DAMGO stimulation.