Prices plus VAT plus shipping costs
Ready to ship today,
Delivery time appr. 5-8 days
- Order number: 7TM0319-SP
- Content: 5 x 20 µl
- Host: Rabbit
MOP Sample Pack consisting of all four available phospho- and one non-phospho-µ-Opioid Receptor Antibodies 5 x 20 µL trial size each. Specifically, this sample pack contains the following antibodies pT370-MOP (7TM0319B), pS375-MOP (7TM0319C), pT376-MOP (7TM0319D) pT379-MOP (7TM0319E) and MOP (non-phos, C-Term) (7TM0319N).
| Alternative Names | MOP, OPRM1, µ-Opioid Receptor, Mu Receptor |
| IUPHAR Target ID | 319 |
| UniProt ID | P35372 (human) P42866 (mouse) P33535 (rat) |
| Western Blot (WB) | 1:1000 |
| Species Reactivity | Human, Mouse, Rat |
| Host / Isotype | Rabbit / IgG |
| Class | Polyclonal |
| Immunogen | Synthetic phospho- and non-phosphopeptides derived from human MOP. |
| Form | Liquid |
| Purification | Antigen affinity chromatography |
| Storage buffer | Dulbecco's PBS, pH 7.4, with 150 mM NaCl, 0.02% sodium azide |
| Storage conditions | short-term 4°C, long-term -20°C |
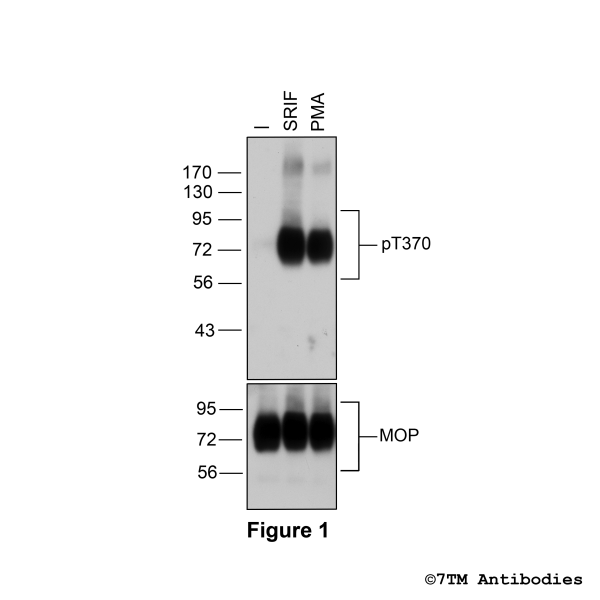
Figure 1. Agonist-induced Threonine370 phosphorylation of the µ-opioid receptor. Upper panel, HEK293 cells stably expressing the µ-opioid receptor (MOP) were either not exposed or exposed to 10 μM DAMGO ([D-Ala2,N-MePhe4, Gly-ol]-enkephalin) or 0.1 μM PMA (Phorbol 12-Myristate 13-Acetate) for 30 minutes. Cells were lysed and immunoblotted with the anti-pT370-MOP antibody (7TM0319B) at a dilution of 1:1000. Lower panel, blot was stripped and reprobed with the phosphorylation-independent anti-MOP antibody (7TM0319N-WB) at a dilution of 1:1000 to confirm equal loading of the gel.
Figure 2. Agonist-induced Serine375 phosphorylation of the µ-Opioid Receptor. Upper panel, HEK293 cells stably expressing the µ-Opioid Receptor (MOP) were either not exposed or exposed to 10 μM DAMGO ([D-Ala2,N-MePhe4, Gly-ol]-enkephalin) or 0.1 μM PMA (Phorbol 12-Myristate 13-Acetate) for 30 minutes. Cells were lysed and immunoblotted with the anti-pS375-MOP antibody (7TM0319C) at a dilution of 1:1000. Lower panel, blot was stripped and reprobed with the phosphorylation-independent anti-MOP antibody (7TM0319N-WB) at a dilution of 1:1000 to confirm equal loading of the gel.
Figure 3. Agonist-induced Threonine376 phosphorylation of the µ-Opioid Receptor. Upper panel, HEK293 cells stably expressing the µ-Opioid Receptor (MOP) were either not exposed or exposed to 10 μM DAMGO ([D-Ala2,N-MePhe4, Gly-ol]-enkephalin) or 0.1 μM PMA (Phorbol 12-Myristate 13-Acetate) for 30 minutes. Cells were lysed and immunoblotted with the anti-pT376-MOP antibody (7TM0319D) at a dilution of 1:1000. Lower panel, blot was stripped and reprobed with the phosphorylation-independent anti-MOP antibody (7TM0319N-WB) at a dilution of 1:1000 to confirm equal loading of the gel.
Figure 4. Agonist-induced Threonine379 phosphorylation of the µ-Opioid Receptor. Upper panel, HEK293 cells stably expressing the µ-Opioid Receptor (MOP) were either not exposed or exposed to 10 μM DAMGO ([D-Ala2,N-MePhe4, Gly-ol]-enkephalin) or 0.1 μM PMA (Phorbol 12-Myristate 13-Acetate) for 30 minutes. Cells were lysed and immunoblotted with the anti-pT379-MOP antibody (7TM0319E) at a dilution of 1:1000. Lower panel, blot was stripped and reprobed with the phosphorylation-independent anti-MOP antibody (7TM0319N-WB) at a dilution of 1:1000 to confirm equal loading of the gel.
Figure 5. Validation of the µ-Opioid Receptor in transfected HEK293 cells. Native HEK293 cells (MOCK) or HEK293 cells stably expressing the µ-Opioid Receptor (MOP) were lysed and immunoblotted with the phosphorylation-independent anti-MOP antibody (7TM0319N-WB) at a dilution of 1:1000.
Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, Schulz S. Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun. 2019 Jan 21;10(1):367. doi: 10.1038/s41467-018-08162-1. PubMed PMID: 30664663; PubMed Central PMCID: PMC6341117.
Miess E, Gondin AB, Yousuf A, Steinborn R, Mösslein N, Yang Y, Göldner M, Ruland JG, Bünemann M, Krasel C, Christie MJ, Halls ML, Schulz S, Canals M. Multisite phosphorylation is required for sustained interaction with GRKs and arrestins during rapid μ-opioid receptor desensitization. Sci Signal. 2018 Jul 17;11(539). pii: eaas9609. doi: 10.1126/scisignal.aas9609. PubMed PMID: 30018083.
Yousuf A, Miess E, Sianati S, Du YP, Schulz S, Christie MJ. Role of Phosphorylation Sites in Desensitization of µ-Opioid Receptor. Mol Pharmacol. 2015 Oct;88(4):825-35. doi: 10.1124/mol.115.098244. Epub 2015 May 12. PubMed PMID: 25969388.
Radoux-Mergault A, Oberhauser L, Aureli S, Gervasio FL, Stoeber M.Subcellular location defines GPCR signal transduction. Sci Adv. 2023 Apr 21;9(16):eadf6059. doi: 10.1126/sciadv.adf6059. Epub 2023 Apr 19. PMID: 37075112; PMCID: PMC10115417.
Just S, Illing S, Trester-Zedlitz M, Lau EK, Kotowski SJ, Miess E, Mann A, Doll C, Trinidad JC, Burlingame AL, von Zastrow M, Schulz S. Differentiation of opioid drug effects by hierarchical multi-site phosphorylation. Mol Pharmacol. 2013 Mar;83(3):633-9. doi: 10.1124/mol.112.082875. Epub 2012 Dec 13. PubMed PMID: 23239825; PubMed Central PMCID: PMC3583494.
Doll C, Pöll F, Peuker K, Loktev A, Glück L, Schulz S. Deciphering µ-opioid receptor phosphorylation and dephosphorylation in HEK293 cells. Br J Pharmacol. 2012 Nov;167(6):1259-70. doi: 10.1111/j.1476-5381.2012.02080.x. PubMed PMID: 22725608; PubMed Central PMCID: PMC3504992.
Doll C, Konietzko J, Pöll F, Koch T, Höllt V, Schulz S. Agonist-selective patterns of µ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol. 2011 Sep;164(2):298-307. doi: 10.1111/j.1476-5381.2011.01382.x. PubMed PMID: 21449911; PubMed Central PMCID: PMC3174411.